| Product Name | Anti-CD8 (Hu) from Mouse (TC8) |
|---|---|
| Description |
Clone TC8 has been developed specifically for routine immunohistochemical (IHC) detection of CD8 in formalin-fixed paraffin-embedded tissue specimen. TC8 has been validated for the identification of CD8 positive tumor infiltrating T cells (TILs) in order to allow the detection of TIGIT in the tumor microenvironment under pathological conditions. The T-cell receptor (TCR) recognizes specific antigenic peptides on the surface of cancer and other target cells presented by HLA-I/β2m complexes. Binding to TCR induces a signalling transduction cascade, leading to execution of cytotoxic T lymphocyte (CTL) functions. Thus, CD8+ T cells are directly involved in antitumor cytotoxic responses, while inhibitory T-cell receptors such as PD-1, CTLA-4 and TIGIT are activated by the immunosuppressive tumor microenvironment with the aim to inactivate tumor-infiltrating lymphocytes (TILs). The most effective current cancer immunotherapies include immune checkpoint inhibition ICI and block T-cell inhibitory receptors. Moreover, effective blockade immunotherapy appears to be associated with the presence of CD8+ T cells.
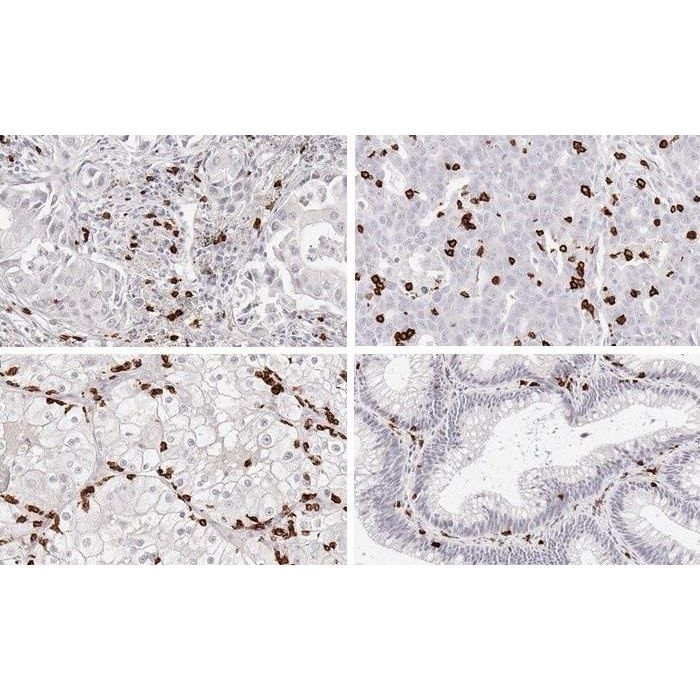
Immunohistochemical application of monoclonal antibody TC8 may provide valuable information for clinical research and poten-tial therapeutic interventions specifically targeting the TIGIT-related tumor immunology checkpoint. Clone TC8 validated for studying tumor infiltrating CD8 positive T cells in FFPE tissues CD8+ T cells play a central role for the killing of cancer cells. They have the ability to infiltrate different human tumors and are engaged in the development of a specific tumor microenvironment. Cancer cells have developed mechanisms to successfully evade the antitumor immune response by generating inhibitory signals through upregulation of the expression of immunosuppressive components. Effective blockade of this interaction is considered as a major factor in the development of cancer immunotherapies. Moreover, preexisting CD8+ T cells seem to predict the efficacy of such immune checkpoint therapies. The T-cell receptor (TCR) recognizes specific antigenic peptides on the surface of cancer and other target cells presented by HLA-I/β2m complexes. Binding to TCR induces a signaling transduction cascade, leading to execution of cytotoxic T lymphocyte (CTL) functions. While CD8+ T cells are directly involved in antitumor cytotoxic responses, the involvement of CD4+ T cells is more indirect, e.g. by their help in priming of CD8+ T cells. In contrast, inhibitory T-cell receptors such as PD-1, CTLA-4 and TIGIT are activated by the immunosuppressive tumor microenvironment with the aim to inactivate tumor-infiltrating lymphocytes (TILs). The most effective current cancer immunotherapies include immune checkpoint inhibition ICI and block T-cell inhibitory receptors. Moreover, effective blockade immunotherapy appears to be associated with the presence of CD8+ T cells. Clone TC8 has been developed specifically for the immunohistochemical (IHC) detection of CD8 in routine FFPE human tissue specimen. TC8 has been validated for the identification of CD8 positive tumor infiltrating T cells (TILs) with the aim to allow an unequaled specific detection of CD8 in the tumor microenvironment. IHC application of monoclonal antibody TC8 may provide valuable information for clinical research and potential therapeutic interventions targeting the tumor immunology checkpoint. (Pictures kindly provided by the Department of Pathology, University Hospital Eppendorf (UKE), Hamburg, Germany) |
| Host | Mouse |
| Clone | TC8 |
| Immunogen | Recombinant peptide of human CD8 |
| Isotype | IgG2a,k |
| Specificity | CD8 |
| Reactivity | Human |
| Applications | IHC |
| Form | Lyophilized, in PBS with 2% BSA, 0.05% NaN3, pH 7.4. Antibody purified from culture supernatant |
| Notes | RUO |
| Background |
Clone TC8 has been developed specifically for routine immunohistochemical (IHC) detection of CD8 in formalin-fixed paraffin-embedded tissue specimen. TC8 has been validated for the identification of CD8 positive tumor infiltrating T cells (TILs) in order to allow the detection of TIGIT in the tumor microenvironment under pathological conditions. The T-cell receptor (TCR) recognizes specific antigenic peptides on the surface of cancer and other target cells presented by HLA-I/β2m complexes. Binding to TCR induces a signalling transduction cascade, leading to execution of cytotoxic T lymphocyte (CTL) functions. Thus, CD8+ T cells are directly involved in antitumor cytotoxic responses, while inhibitory T-cell receptors such as PD-1, CTLA-4 and TIGIT are activated by the immunosuppressive tumor microenvironment with the aim to inactivate tumor-infiltrating lymphocytes (TILs). The most effective current cancer immunotherapies include immune checkpoint inhibition ICI and block T-cell inhibitory receptors. Moreover, effective blockade immunotherapy appears to be associated with the presence of CD8+ T cells. Immunohistochemical application of monoclonal antibody TC8 may provide valuable information for clinical research and poten-tial therapeutic interventions specifically targeting the TIGIT-related tumor immunology checkpoint. |
| Supplier | Dianova GmbH |
All Research Products are sold for laboratory RESEARCH USE ONLY and ARE NOT TO BE USED FOR HUMAN OR ANIMAL THERAPEUTIC OR DIAGNOSTIC APPLICATIONS. The information presented is believed to be accurate; however, said information and products are offered without warranty or guarantee since the ultimate conditions of use and the variability of the materials treated are beyond our control. Nothing disclosed herein is to be construed as a recommendation to use our products in violation of any patents. ARP American Research Products, Inc. does not submit its products for regulatory review by any government body or other organization, and we do not validate them for clinical, therapeutic or diagnostic use, or for safety and effectiveness. You are solely responsible for making sure that the way you use the products complies with applicable laws, regulations and governmental policies and for obtaining all necessary approvals, intellectual property rights, licenses and permissions that you may need related to your use. Under no circumstances shall ARP American Research Products, Inc. be liable for damages, whether consequential, compensatory, incidental or special, strict liability or negligence, breach of warranty or any other theory arising out of the use of the products available from ARP American Research Products, Inc. Nothing contained herein warrants that the use of the products will not infringe on the claims of any patents covering the product itself or the use thereof in combination with other products or in the operation of any process. ARP American Research Products, Inc. disclaims any and all representations or warranties of any kind whatsoever, express or implied, including without limitation any implied warranties of merchantability or fitness for a particular purpose, of non-infringement, or regarding results obtained through the use of any product, whether arising from a statute or otherwise in law or from a course of performance, dealing or usage of trade.