Immune Checkpoints
Immune Checkpoints
Immune Checkpoint Product Focus
The immune system must maintain a balance in order to allow appropriate responses to infection while preventing inappropriate responses to self-antigens. There are numerous pathways which develop and maintain this balance and immune checkpoint pathways have become popular targets for therapeutic drug development.
Immune checkpoints are pathways which help regulate immune responses and can be either inhibitory or stimulatory. Inhibitory pathways are critical to maintain self-tolerance and act to prevent autoimmune disease and help to turn off immune responses. Stimulatory pathways are necessary to activate the immune response to fight infection.
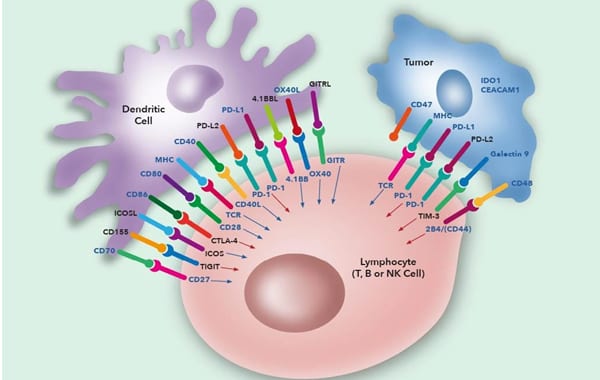
Perhaps the best illustration of this is the fact that full T cell activation can only occur after 2 stages:
The first stage involves binding of the T-cell receptor on CD4+ helper T cells or CD8+ cytotoxic T cells with antigen held in complex with MHC class I on normal or tumour cells or MHC class II on antigen presenting cells (APC). CD4 or CD8 molecules on the T cell also bind to stabilise this complex with CD4 binding to class II and CD8 to class I. In addition to the initial binding of TCR and MHC there are a variety of additional signals required to fully activate the T cells.
The second stage includes binding of the co-stimulatory molecule CD28 on helper T cells with the B7.1 (CD80) and/or B7.2 (CD86) molecules on the APC to stimulate T cell proliferation. Control of this proliferative response is controlled by the induction of the negative regulator CTLA-4 (CD152) on T cells which competes for binding to B7.1/B7.2 to reduce stimulatory signalling and turn off the immune response. Cytotoxic CD8 T cells are less dependent on the CD28/CTLA-4 pathway but do utilise other pathways such as CD70/CD27 and 4-1BB (CD137)/4-1BBL. Other inducible molecules such as ICOS, 4-1BB and OX40 can also be expressed on T cells and function to enhance or maintain immune responses.
In recent years, immune checkpoint pathways have become an important target for cancer immune therapy (Immuno-Oncology). This is due to the ability of cancer cells to evade the immune response by manipulating immune checkpoint pathways to switch off the immune response.
For example, tumour cells have been found to express ligands to molecules such as PD-1 expressed on activated immune cells. Binding of inhibitory ligands to receptors on activated immune cells helps to downregulate and switch off the immune response. To overcome this, cancer therapies have focused on blocking these inhibitory pathways using immune checkpoint antibodies such as anti-CTLA-4, anti-PD1 and anti-PD-L. Indeed the 2018 Nobel Prize in Physiology or Medicine was won by James P. Allison and Tasuku Honjo for their work on cancer immunotherapy.
Ipilimumab (anti-CTLA-4 antibody) was the first immune checkpoint inhibitor to be given FDA approval (2011) and has been swiftly followed by Pembrolizumab and Nivolumab (anti-PD1) and Atezolizumab, Avelumab and Durvalumab (anti-PD-L1).
ARP Inc provide a variety of immune checkpoint antibodies to support your research, including anti-mouse CTLA-4 antibody and anti-mouse PD1.